by Charlotte M. Bird 1
Introduction:
Imagine you are an avid fossil hunter and have just dug up a skull of an extinct vertebrate. You are the first human ever to see it. Not only is that amazing, but you are also at the start of a journey into discovering how this organism lived: whether it was diurnal (active during the day) or nocturnal, whether it hunted above ground or burrowed, had poor vision or an exceptional sense of smell. Despite the millions of years that may have passed, the growing field of virtual
What are digital endocasts?
Virtual Palaeontology is the non-destructive study of fossils using digital methods, enabling, for example, both analyses of external skull features, alongside
For the creation of digital endocasts, computed tomography (CT) scans of fossils (comprised of potentially thousands of images of thin slices through it) are used. These pictures are loaded into imaging software such as Avizo or SPIERS. In the
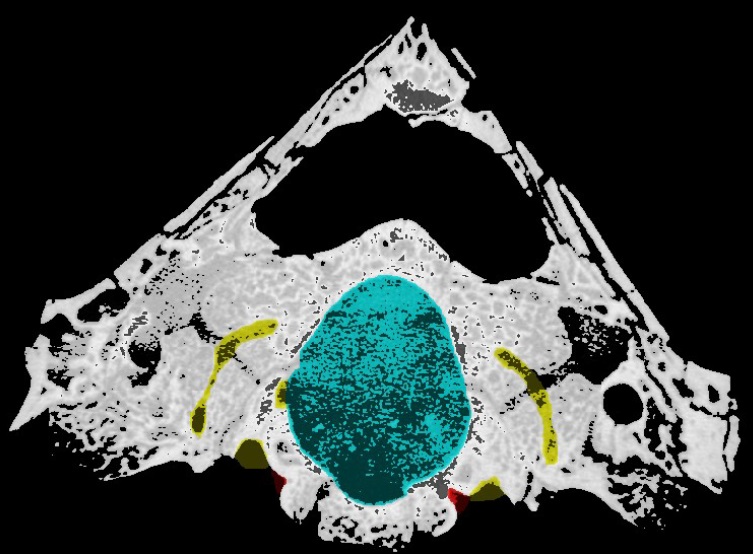
What can cranial endocasts tell us?
To put endocasts into context, a recent study considered the changing endocranial anatomy of the skulls of cynodonts, a group of therapsids (fossil reptiles) that appeared during the late Permian period (around 259 million to 252 million years ago) and expanded during the Triassic period (252 million to 201 million years ago), with one branch leading to modern mammals. Understanding how the brain of mammals evolved from those of primitive cynodonts, developing the ability to carry out the complex functions that make modern mammals distinct, is of great importance to palaeoneurology — a field of research that has focused on the evolution of the human brain from those of our primate ancestors.
3D models of cynodont brains (Fig. 2) enable palaeontologists to describe new aspects of the anatomy of little-known species, such as Thrinaxodon
- 3D point cloud analysis in CloudCompare, which uses a colour scale to map variations in shape between 3D models, allowing for variations in brain shape to be identified and mapped.
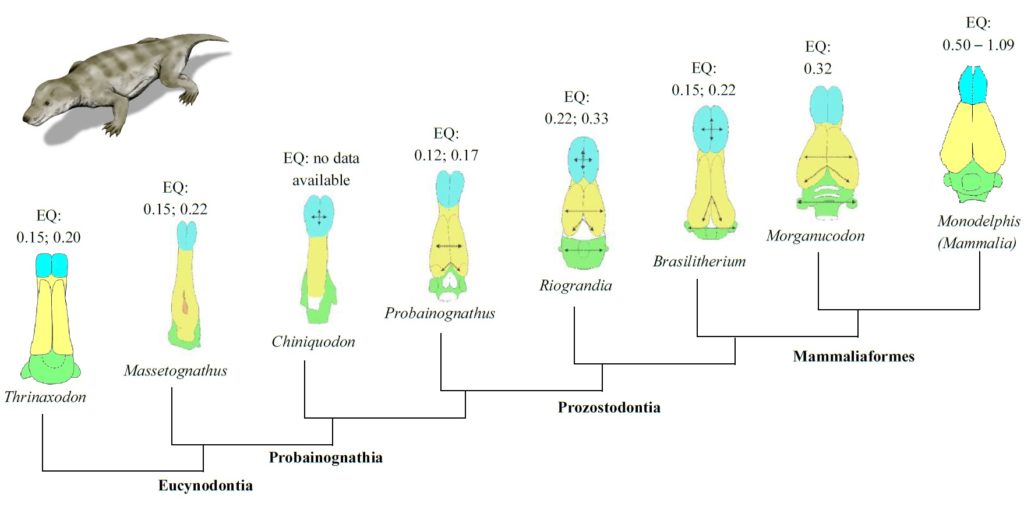
- Calculation of encephalisation quotients, in which brain volumes determined from the 3D models are used to estimate a relative level of cognitive ability (intelligence) for a species relative to other species through time. Higher encephalisation quotient values are associated with social interactions and foraging behaviour, so the quotient offers information about ways of life.
- Assessing hearing capability, or auditory acuity, which can be assessed through measurements of the length of a cavity in the inner ear called the cochlea.
- Measuring the shape and size of semi
–circular canals of the inner ear, which provide information on an individual’s agility, once more indicating behavioural patterns.
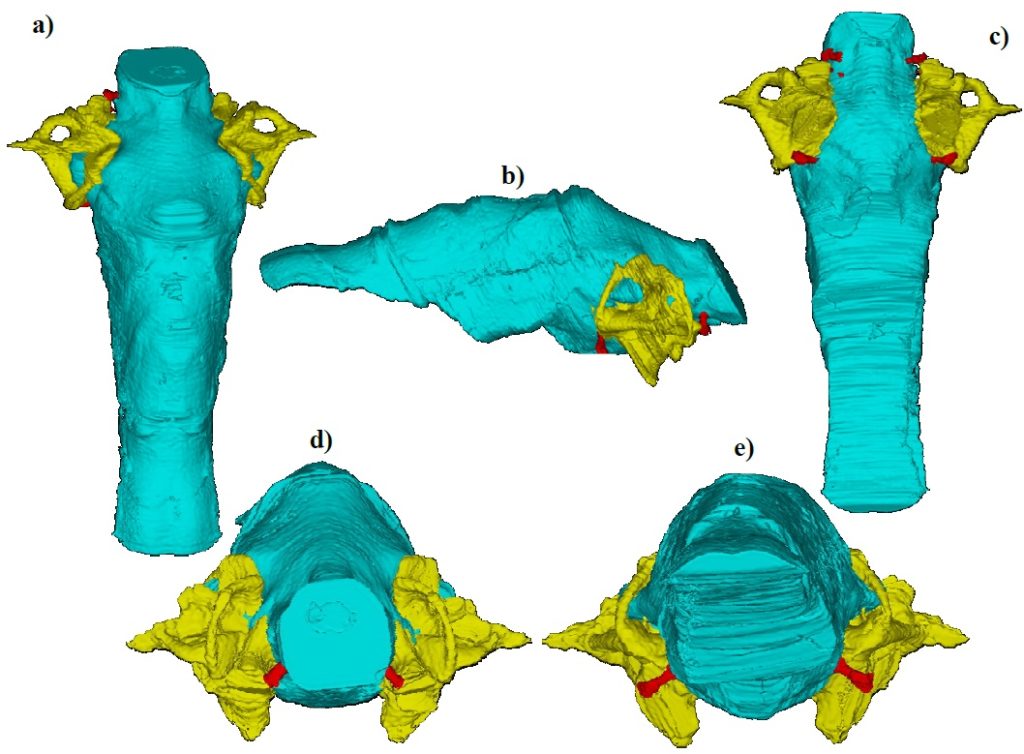
From an in-depth study of cranial endocasts, three stages of development have been identified in the mammal brain over time (Fig. 3). First,
Endocasts are pretty great …
You can access your specimens anywhere in the world, at any time, as long as you have your data sets. Digital reconstruction saves your data for the future, in case the original specimen is lost or damaged. CT scanning the fossil also means that you do not have to destroy the original by grinding away thin layers to take photographs of cross-sections to make a 3D model.
More importantly, however, cranial endocasts help to overcome some of the bias in the fossil record. Soft tissues are rarely preserved, yet can provide some of the most pivotal information about form, function and mode of life for extinct organisms. Based on the assumption that the brain followed the shape of the
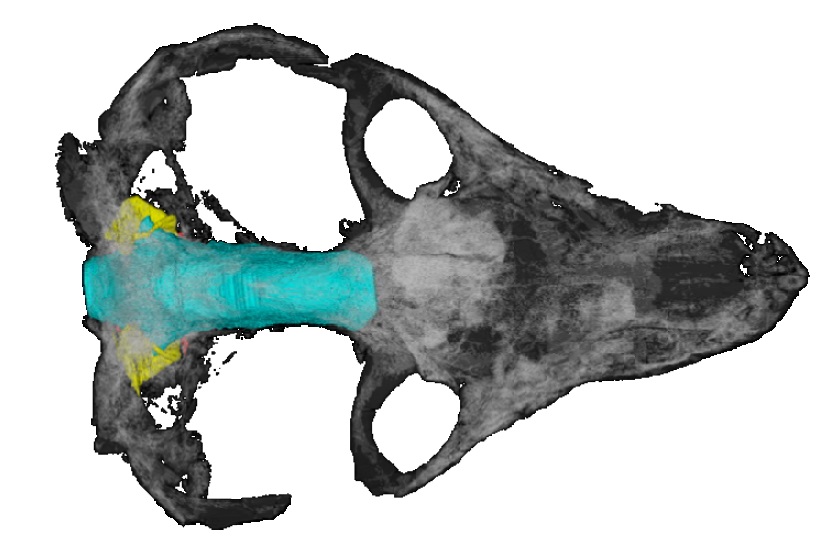
… But they have their limitations
Despite endocasts, and virtual palaeontology itself, providing fantastic leaps forward in fossil research, digital reconstructions do have some limitations. First, the assumption made during endocast creation that the intracranial space correlates directly with the original soft-tissue structures of the brain is problematic. Modern mammals have additional fluid and tissues in the intracranial space (for example, meninges, blood vessels and nerves). The external surface of the mammal brain is also known to be convoluted with ridges (gyri) and grooves (sulci), but no direct impression of this can be discerned on the braincase. Therefore, palaeoneurological studies are limited to external features of the endocasts and 3D models also do not provide any direct information about the brain’s internal structures (such as neuron morphology, density
Furthermore, the fossilisation process itself causes a bias in the types of endocast that can be produced. Skulls are often deformed or
Technology itself can be a problem. Standard CT scanning takes images using X-rays; these have fairly low resolution, so it can be difficult to see fine detail. Fossils scanned with micro-CT will produce much higher resolution images than X-ray scanning, making the former preferable for acquiring fine detail data sets to study.
And finally, the biggest limitation to endocasts can be the researcher. Everyone has their own opinions on what they are looking at, depending on prior knowledge, which ultimately affects what is included within the mask that defines the model. Small differences during endocast construction could lead to potentially significant morphological and volumetric discrepancies, affecting all subsequent analyses. The specimens chosen for
Conclusions
Endocasts are invaluable to reconstructing soft tissues lost to time, and to inferring how these anatomical features may have impacted the manner in which the individual lived, and how behavioural patterns may have changed during the evolution of a species or a larger part of the mammalian lineage. 3D models provide the closest possible approximation palaeontologists can obtain of what lay beneath the surface of fossil skulls, and provide valuable information on the development of the mammalian brain through time. Moreover, cranial endocasts offer possibilities for determining how sensory capabilities impacted upon the size and shape of various brain regions.
Whilst reconstruction and analysis methods have their limitations, further research will continue to fine-tune these methods, providing an exciting future for the emerging field of virtual palaeontology — aiding not only fossil
Suggestions for further reading:
Cox, P. G. & Jeffery, N. Semicircular canals and agility: the influence of size and shape measures. Journal of Anatomy 216, 37–47 (2010). (DOI: 10.1111/j.1469-7580.2009.01172.x)
Gleich, O., Dooling, R. J. & Manley, G. A. Audiogram, body mass and basilar papilla length: correlations in birds and predictions for extinct dinosaurs. Naturwissenschaften, 92, 595 – 598 (2005). (DOI: 10.1007/s00114-005-0050-5)
Jasinoski, S. C., Abdala, F. & Fernandez, V. Ontogeny of the Early Triassic cynodont Thrinaxodon liorhinus (Therapsida): cranial morphology. The Anatomical Record 298, 1440–1464 (2015). (DOI: 10.1002/ar.23116)
Kemp, T. S. The Origin and Evolution of Mammals. (Oxford University Press, 2005).
Macrini, T. E., Rowe, T. & VandeBerg, J. L. Cranial endocasts from a growth series of Monodelphis domestica (Didelphidae, Marsupialia): a study of individual and ontogenetic variation. Journal of Morphology 268, 844–865 (2007). (DOI: 10.1002/jmor.10556)
Rodrigues, P. G., Ruf, I. & Schultz, C. L. Digital reconstruction of the otic region and inner ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. Journal of Mammalian Evolution 20, 291–307 (2013). (DOI: 10.1007/s10914-012-9221-2)
Rodrigues, P. G., Ruf, I. & Schultz, C. L. Study of a digital cranial endocast of the non-mammaliaform cynodont Brasilitherium riograndensis (Later Triassic, Brazil) and its relevance to the evolution of the mammalian brain. Paläontologische Zeitschrift 88, 329–352 (2014). (DOI: 10.1007/s12542-013-0200-6)
Rodrigues, P. G., Martinelli, A. G., Schultz, C. L., Corfe, I. J., Gill, P. G., Soares, M. B. & Rayfield, E. J. Digital cranial endocast of Riograndia guaibensis (Late Triassic, Brazil) sheds light on the evolution of the brain in non-mammalian cynodonts. Historical Biology 30, 1–18 (2018). (DOI: 10.1080/08912963.2018.1427742)
Rowe, T. B., Macrini, T. E. & Luo, Z.-X. Fossil Evidence on the Origin of the Mammalian Brain. Science 332, 955–957 (2011). (DOI: 10.1126/science.1203117)
1School of Geography, Earth and Environmental